Manufacturer Good Price Oxalic Acid CAS:144-62-7
Applications of Oxalic Acid
1. Oxalic acid can be mainly used as reducing agent and bleaching agent, mordant for dyeing and printing industry, also used in refining rare metal, the synthesis of various oxalate ester amide, oxalate and grass, etc.
2. Used as analytical reagent.
3. Used as laboratory reagents, chromatography analysis reagent, dye intermediates and standard material.
4. Oxalic acid is mainly used for producing drugs such as antibiotics and borneol and solvent for extracting the rare metal, reducing agent and dye, tanning agent, etc. In addition, oxalic acid can also be used for the synthesis of various kinds of oxalate ester, oxalate, and oxamide with diethyl oxalate, sodium oxalate and calcium oxalate having the largest yield. Oxalate can also be used for the production of cobalt-molybdenum-alumina catalyst, cleaning of metal and marble as well as the bleaching of textiles.
Agricultural Uses:Oxalic acid, (COOH)2, also called ethanedioic acid, is a white, crystalline solid, slightly soluble in water. It is a naturally occurring highly oxidized organic compound with significant chelating activity. It is strongly acidic and poisonous, produced by many plants like sorrel (sourwood), the leaf blades of rhubarb, bark of eucalyptus and many plant roots. In plant cells and tissues, oxalic acid gets accumulated as either sodium, potassium or calcium oxalate, of which the latter occurs as crystals. In turn, salts of oxalic acids enter the bodies of animals and human beings, causing pathological disorders, depending upon the amount consumed. Many species of fungi like Aspergillus, Penicillium, Mucor, as well as some lichens and slime moulds produce calcium oxalate crystals. Upon the death of these microorganisms, plants and animals, the salts get released into the soil, causing some amount of toxicity. However, oxalate-degrading microbes, called Oxalobacter formigenes, decrease oxalate absorption in animals and humans.
Oxalic acid is the first of a series of dicarboxylic acids. It is used (a) as a bleaching agent for stains like rust or ink, (b) in textile and leather production, and (c) as monoglyceryl oxalate in the production of ally1 alcohol and formic acid.
Specification of Oxalic Acid
|
Compound |
Specification |
|
Content |
≥99.6% |
|
Sulfate (In S04), % ≤ |
0.20 |
|
Burning Residue, % ≤ |
0.20 |
|
Heavy metal (In Pb), % ≤ |
0.002 |
|
Iron (In Fe), % ≤ |
0.01 |
|
Chloride (In Ca), % ≤ |
0.01 |
|
Calcium (In Ca), % ≤ |
0.01 |
Packing of Oxalic Acid
25KG/BAG
Storage: Preserve in well-closed, light-resistant, and protect from moisture.


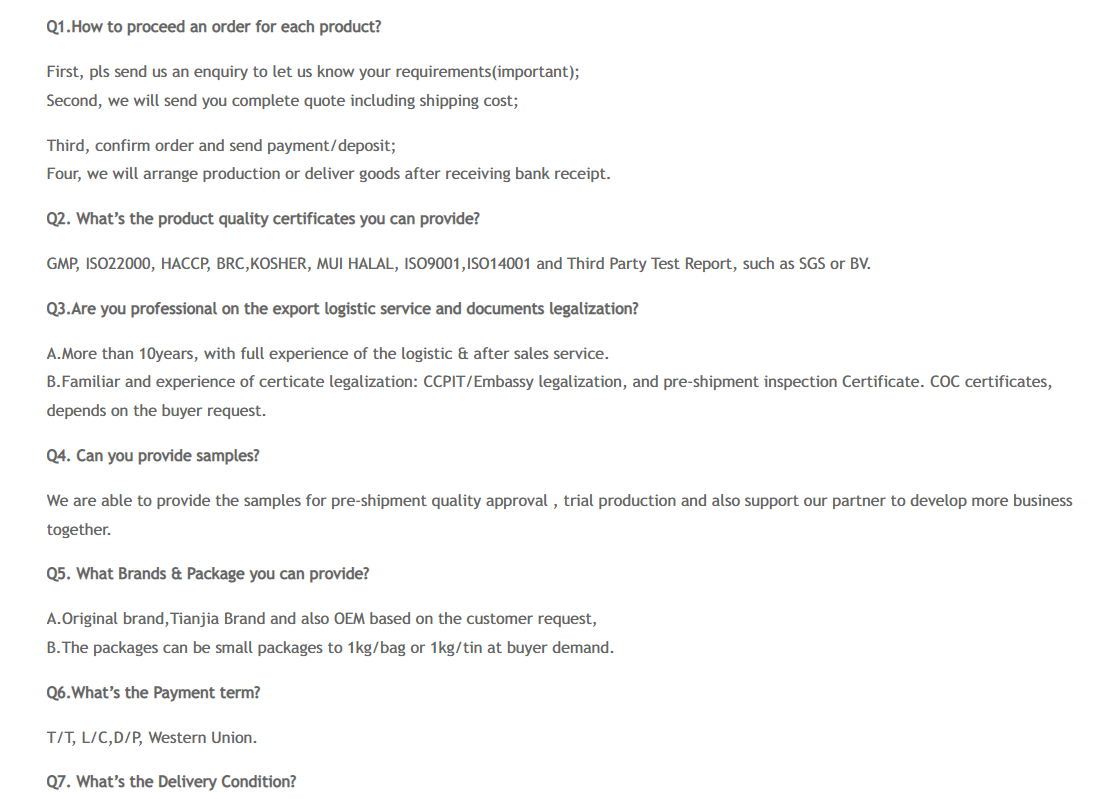
Our Advantages
300kg/drum
Storage: Preserve in well-closed, light-resistant, and protect from moisture.
FAQ